
The most advanced luxurious and clinically affected EECP device in the world.
Soulaire® Corporation has exclusive worldwide distribution rights for its newest state-of-the-art FDA cleared circulation enhancement device that is now available in the marketplace.


Soulaire® EECP device is a safe, effective, non-invasive and clinically proven therapy that enhances your blood circulation.
The therapy comprises of synchronized external pressure applied on the lower limbs during the diastole phase of the heart cycle.
Soulaire® ECP device is cleared by the U.S. Food and Drug Administration (FDA) a non-invasive medical devices used as an adjunctive treatment for patients who suffer from coronary artery disease or ischemic heart failure. CMS (Centers for Medicare and Medicaid Services) reimbursement coverage has been provided for the use of ECP since 1999 for patients who have been diagnosed with disabling angina (Class III or Class IV, Canadian Cardiovascular Society Classification or equivalent classification) and whom are not readily amendable for interventional procedures.
Soulaire® is intended for the treatment of chronic stable angina that is refractory to optimal anti-anginal medical therapy and without options for revascularization. In addition, it is intended for use in healthy patients to provide improvement in vasodilation, and increased blood flow. It is intended for use under the oversight of a healthcare professional.
Soulaire Systems specializes in a unique cutting-edge technology that stems from the original EECP® device, the only clinically blinded studied device in the world.
Soulaire has exclusive worldwide distribution rights for its newest FDA cleared circulation enhancement device.

How Soulaire System works
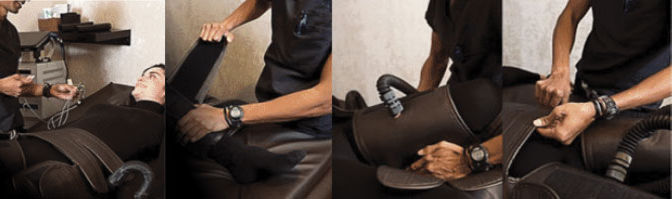
Soulaire systems is an FDA-cleared, non-invasive therapy that works as you lie on a comfortable bed. You’re connected to a 3-lead EKG monitor. Three large blood pressure-like cuffs wrap around your calves, thighs and buttocks.
Working with the rhythm of your heart, the cuffs sequentially inflate when your heart is at rest (diastole). When your heart pumps (systole), the cuffs deflate. These cuffs squeeze the blood from your lower extremities to your heart. It creates the mechanism of a second heart while decreasing the workload on the heart, your blood pressure and heart rate.
Here is a step-by-step look at the process of this safe and non-surgical therapy:
Key Features
- New Quiet Technology
- Trinity Pneumatic Delivery System
- Treatment Mattress
- Single Plethysmographic Probe
- Welded steel frame construction
Legacy and History of EECP
The first EECP device was developed in the late 1950s at Harvard University by Dr. Clifford Birtwell and his associates, who pioneered counterpulsation techniques.
This device utilized hydraulic compression to enhance blood flow during the diastolic phase of the heartbeat. Later, Stony Brook University commenced research with 18 individuals suffering from end-stage cardiovascular disease, aiming to evaluate the efficacy of EECP therapy in this population.
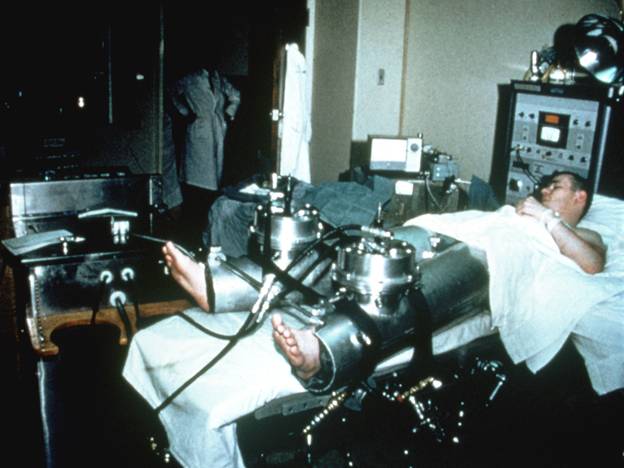
1950s